Optical microscopy of fluorescence microscopy is a special form. It uses the ability of a target wavelength of light to excite a fluorescent dye that emits light. The benefits of protein can be marked by antibody staining or fluorescent protein-labeled fluorescent dyes. It allows the determination of the distribution of a single molecular species, its amount and its localization within the cell. In addition, co-localization and interaction studies can be performed, observed ion concentrations, using reversible binding to dyes such as Ca2+ and furan-2 and endocytosis and extracellular secretion of cellular processes, as observed. Today, it can even be divided into particles with the help of the image, the resolution of the fluorescence microscope
Stokes shift
An important feature of fluorescence is the Stokes shift. It describes the difference in energy levels of an exciting and emitted photon. Photons emitted by fluorescence are an exciting photon with a larger wavelength ratio. This is due to the fluorescent dye that is excited, but the energy of the photons emitted before is released into the surrounding environment. The resulting wavelength shift makes it possible to distinguish between excitable and outgoing light. The energy of absorption and emission of a molecular species that can be considered as a specific feature.
Fluorescence microscope
An upright or inverted fluorescence microscope () is similar to an ordinary optical microscope, except that the laser is used for monochromatic light or mercury or xenon arc lamps to provide illumination like a vibrant and powerful light source. In addition, it also contains an excitation filter and a transmission filter. Excitation filter transmission is only able to excite the sample with its specific dye light. The light emitted by the sample passes through the emission filter before it reaches the detector. Light emitted by the sample. Transmittance of light with only one wavelength, such as an emission filter of light emitted by a sample
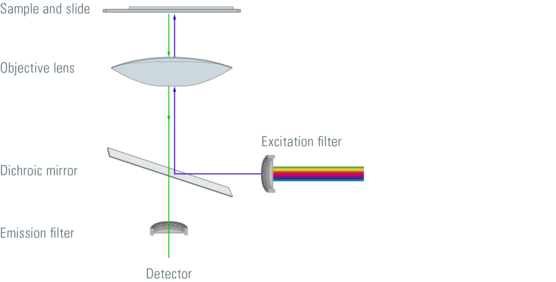
Figure 1: Light path through a fluorescence microscope
In recent years, light-emitting diodes (light-emitting diodes) have also been used as light sources for fluorescent microscopes. The wavelength of light emitted by the LED depends on the material used for production. However, in most cases, an excitation filter is required because the LED typically emits light over a relatively wide range of wavelengths.
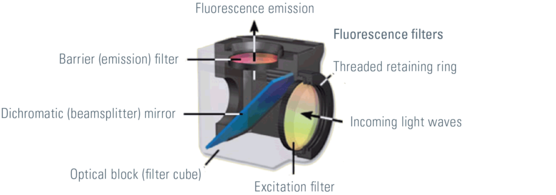
Most fluorescence microscopes are epi-fluorescent microscopy. The illuminator and objective lens are positioned on the same side of the test piece and the light passing through the sample does not pass. In addition to the excitation and emission filters, a dichroic mirror is required for this fluorescence microscope. A dichroic mirror that passes light of a target wavelength while other wavelengths of light are reflected. Filters and dichroic mirrors are often inserted together into the cube in the filter.
The excitation light passes through the excitation light filter and the dichroic mirror. This reflects the passage of light through the objective lens to the sample. Specimens in the specimen and photons emitted by fluorescent dyes. This emitted light passes through an objective dichroic mirror. The emitted light has an appropriate wavelength and can pass. The excitation light reflected by the sample cannot pass through the dichroic mirror and is blocked. If the excitation light can pass through the dichroic mirror it will block when it reaches the emission filter. The light of the detector's emission filter that can be measured passes.
Different types of filters are used in fluorescence microscopy. Bandpass, long pass and short pass filters can be distinguished. The wavelength bandpass filter of the transmitted frequency band, while light having a larger or smaller wavelength will be blocked. Long pass and short pass filter edge filters. Long-wavelength optical transmission of long-pass filters. Light with a wavelength above the cutoff of the target will not pass. In contrast, short-pass filters transmit short wavelengths and prevent long
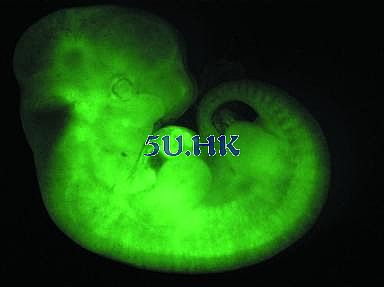
Figure 3. Green fluorescent protein transgenic mouse embryo

Figure 4: Fluorescence in situ hybridization of chromosomes. Red: TRITC, blue: CY5, green: FITC
Different techniques in fluorescence microscopy
Fluorescence microscopy is widely used and provides great specificity. The various techniques make it possible to solve different problems and even circumvent the diffraction limit described by Abbe.
Localization of molecular species can be determined with staining of organelles such as cytoskeleton or membranes. Confocal laser scanning microscopy (CLSM) allows it to observe areas of the sample that have no signal from the outside focal plane and allows for optical sectioning. Total Internal Reflection Microscopy (TIRF) is a technique that allows observation of thin areas close to the cell surface. Fluorescent dyes excited in an evanescent field in this respect.
One technique for observing the dynamics of a molecular species is fluorescence bleaching recovery (FRAP). The diffusion of photobleaching and unbleached molecules of fluorescent dyes in a restricted area can be measured by entering this area. Fluorescence energy transfer (FRET) microscopes allow for interactive studies. An excited donor chromophore transfers energy to the receptor to excite it. If both are brought together very closely, this is the only possibility. If these dyes are coupled to different proteins, they are only capable of transmitting energy, emitting fluorescent proteins if they interact with each other.
Techniques that enable stimulated emission loss (STED) microscopy, ground state consumption (GSD) microscopy, single molecule and ground state depletion microscopy from single molecule returns (GSDIM), photosensitive localization microscopy (PALM) and random sub-high resolution image optics Reconstruction microscope (STORM). Submicroscopes used in these techniques are also known as nanoscopes because they solve images at the nanoscale.
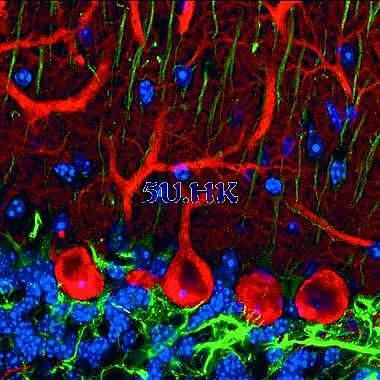
Figure 5: Pukinje cells, sagittal triple-labeled mouse cerebellar cortex. Red: anti-calbindin-D28k/Cy3, green: blue anti-GFAP/Cy5: Hearst 33258
Figure 6: Mouse tissue section, autofluorescence, fluorescence lifetime microcsopy (FLIM)
Pet Crystal Screen,Pet Crystal Alr Screen,Ust Alr Pet Crystal Screen,Laser Projection Screen
Dongguan Aoxing Audio Visual Equipment CO.,Ltd , https://www.aoxing-whiteboard.com